The limitations of lithium-ion batteries are well known at this point, so a number of automakers are exploring alternatives such as lithium-air technology.
Honda is taking a different approach as their scientists have teamed up with researchers from the California Institute of Technology and NASA’s Jet Propulsion Laboratory to develop a new battery chemistry that promises to be a significant improvement over current battery technology.
While the details will probably make your eyes glaze over, Honda says the research paves the way for “high energy-density batteries capable of meeting rapidly growing energy storage needs by overcoming the current temperature limitations of fluoride-based battery technology and by demonstrating the room-temperature operation of fluoride-ion based energy cells.”
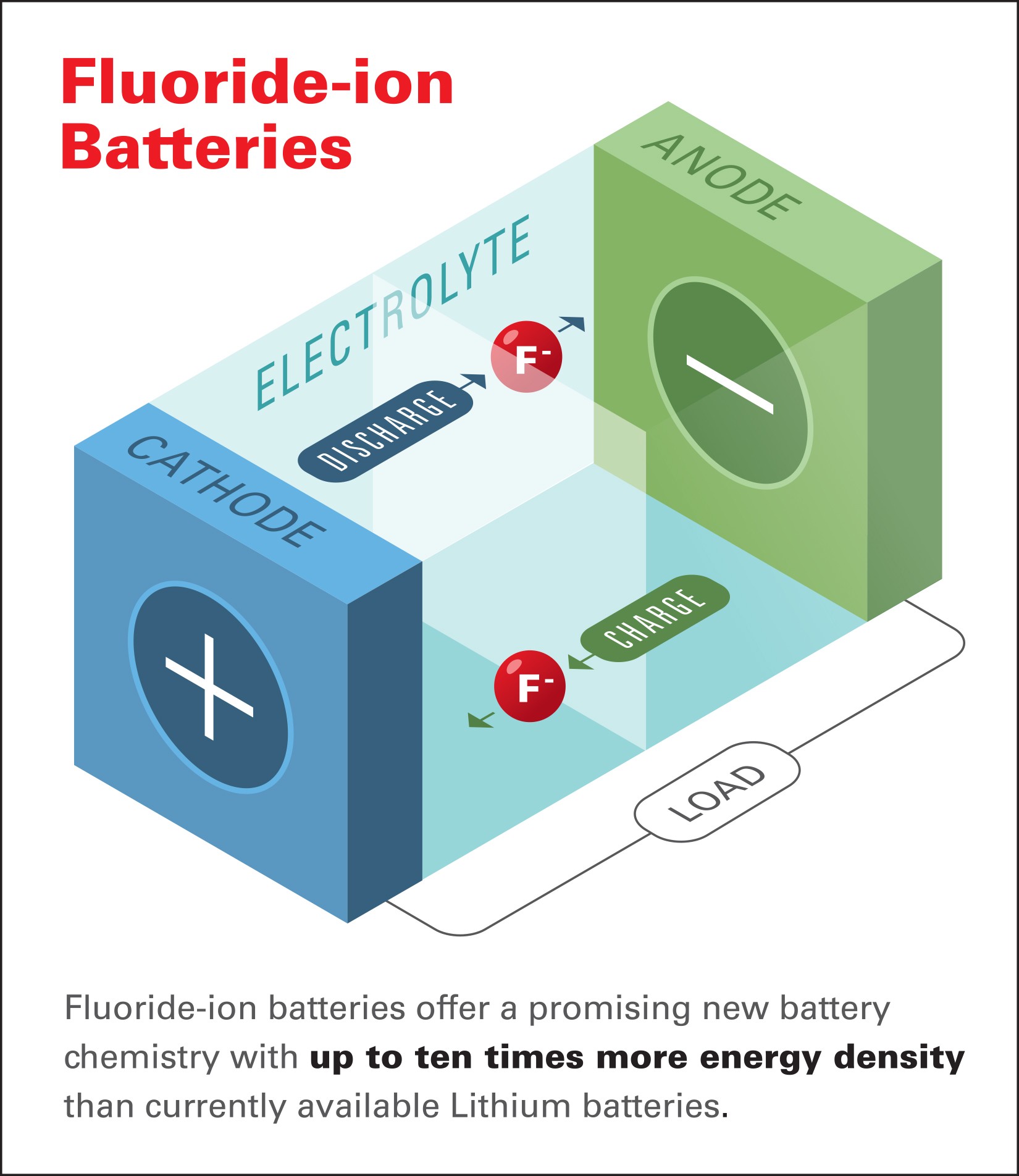
Putting aside the science mumbo jumbo for a minute, the chief scientist of the Honda Research Institute said “Fluoride-ion batteries offer a promising new battery chemistry with up to ten times more energy density than currently available lithium batteries.” That’s a huge improvement and fluoride-ion batteries have the potential to be significantly lighter than lithium-ion batteries while also delivering a vastly improved range when used in electric vehicles.
Dr. Christopher Brooks also noted fluoride-ion batteries are safer than lithium-ion batteries as they don’t suffer from overheating issues. Furthermore, the batteries are more environmentally friendly than today’s lithium-ion batteries.
Of course, fluoride-ion batteries have their drawbacks as Honda noted current solid-state fluoride ion-conducting batteries need to operate at temperatures above 302 F° (150° C) to make the electrolyte fluoride-conducting. This has been a “significant challenge,” but the research team found a way to create a fluoride-ion electrochemical cell capable of operating at room temperature.
Honda says this breakthrough was achieved by using a “chemically stable liquid fluoride-conducting electrolyte with high ionic conductivity and a wide operating voltage.” The scientists also developed a unique electrolyte using “dry tetraalkylammonium fluoride salts dissolved in an organic, fluorinated ether solvent.” This is paired with a composite cathode which features a core-shell nanostructure made of copper, lanthanum and fluorine. Thanks to this setup, the researchers were able to successfully demonstrate reversible electrochemical cycling at room temperature.